Hexane and n-Hexane
Hexane and n-Hexane are hydrocarbons with the same chemical formulas but distinct structures, resulting in various properties and applications. Hexane is an alkane with straight chains with the molecular formula of C6H14 and n-hexane is an isomer of the hexane and shares the same molecular formula however having an entirely different arrangement of carbon atoms. Because of their low toxicity and superior chemical properties for solvents, hexane as well as n-hexane are extensively used in a range of fields.
Hexane is used in extracting of vegetable oils for cleaning purposes and also as solvents in the manufacturing of cosmetics, pharmaceuticals and other chemicals. However, it is used primarily in lab settings as a non-polar solvent used in chemical extraction and reactions. But, despite their utility each chemical poses dangers to health, especially with regard to neurotoxic and respiratory effects which require strict safety precautions as well as regulations for their handling and use.
Importance and common uses of both chemicals
Importance and Common Uses of Hexane:
Extracting Vegetables: Oils Hexane is an essential solvent utilized in the extraction of vegetable oils using the seeds as well as nuts. It effectively dissolves oil out of plant materials, making it an indispensable component of the manufacturing of margarine, cooking oils, and various food items.
Industrial Solvents: The outstanding solvent properties make it a necessity in a myriad of industrial processes for dissolving glues, resins and waxes.
Pharmacies Hexane is a solvent for pharmaceutical manufacturing processes which aids in extracting active substances from plants as well as being an ingredient in formulations for drugs.
Cosmetics: Its capability to dissolve fats and oils is a major advantage for the creation of cosmetics, which includes creams, lotions, as well as makeup products.
Cleaning Agent: Because of its low toxicity as well as a rapid growth rate of evaporation, hexane can be used as a cleaner in the electronic and precision machinery industries.
Important and common uses of the n-Hexane chemical:
Laboratory Solvent: n-Hexane as a non-polar solvent is frequently used in laboratories for a myriad of reasons like extraction, purification, as well as techniques for chromatography.
Organic Synthesis: Organic Synthesis is used as a medium of reaction for organic synthesis. It facilitates interactions between nonpolar organic compounds.
Industrial Cleaning: In certain industries, nhexane is used for the purpose of cleaning equipment and machinery due to its capability to dissolve grease, oils and various organic pollutants.
Adhesives and Glues: chemical is used in glues and adhesives, resulting in strong bonding properties to various substances.
Polymer Processing: n-Hexane has been utilized in the manufacturing of particular polymers. It assists in helping them melt and mold into different types of products, such as film and fibers.
Hexane and N-hexane are both essential chemical compounds that have applications in diverse sectors. The prominent use of hexane in pharmaceuticals, industrial solvents and cosmetics, as well as its importance in research and development organic synthesis, as well as industrial cleaning highlights the importance of these chemicals in contemporary processes and production.
Definition of Hexane
Hexane is an organic chemical compound that is classified as an alkane. It is specifically, a straight-chain alkane that has the molecular formula of C6H14. It is part of the family of hydrocarbons, made up of carbon (C) and hydrogen (H) atoms alone and characterized by a linear arrangement of carbon atoms inside the molecular structure.
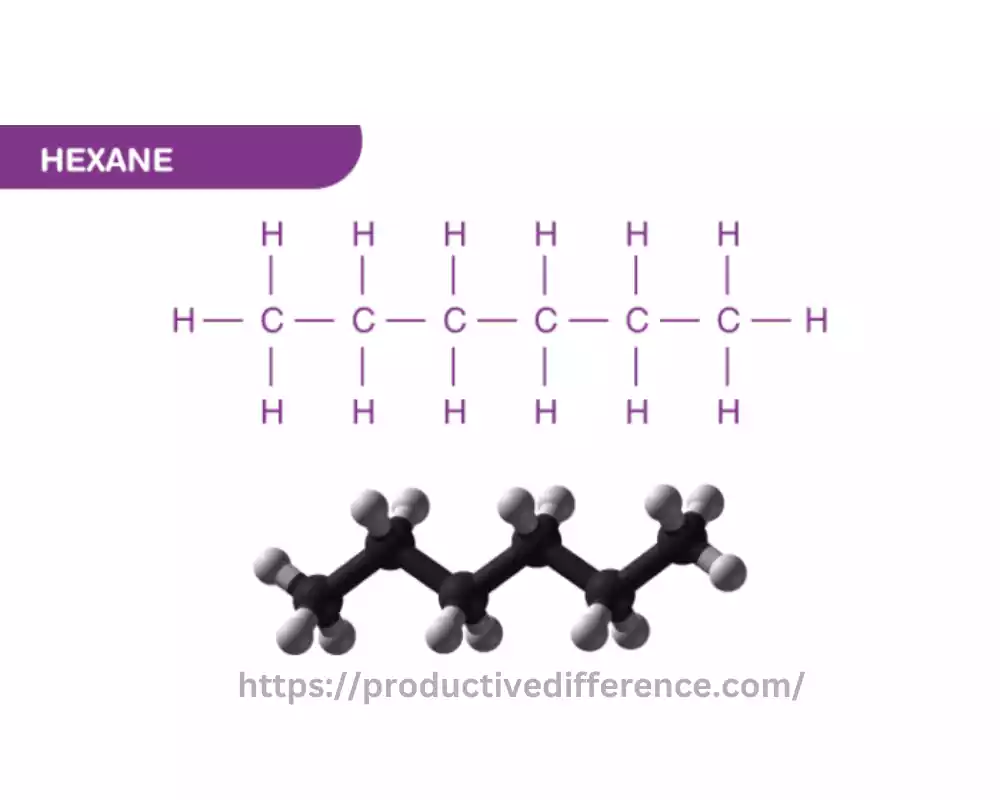
Hexane is a clear, colorless, and smiling liquid at room temperatures and it is largely insoluble in water. It is frequently utilized in many sectors and uses, like a solvent for the extraction from oil, for the manufacture of adhesives, pharmaceuticals, and paints, as well as a cleaning agent for specific industrial procedures. But, exposure for long periods to hexane may cause health hazards, especially in the respiratory and nervous systems. Therefore, the use of safety precautions in handling the chemical.
Definition of n-Hexane
The organic compound n-Hexane is a compound and is an isomer Hexane. It shares the same molecular formula C6H14. It has distinct structural arrangements of carbon atoms as compared to the straight-chain counterpart of hexane. This is why the n-hexane structure is unique. It consists of six carbon atoms within the linear chain. This makes it unique from other hexane isomers that are branched, like the cyclohexane form.
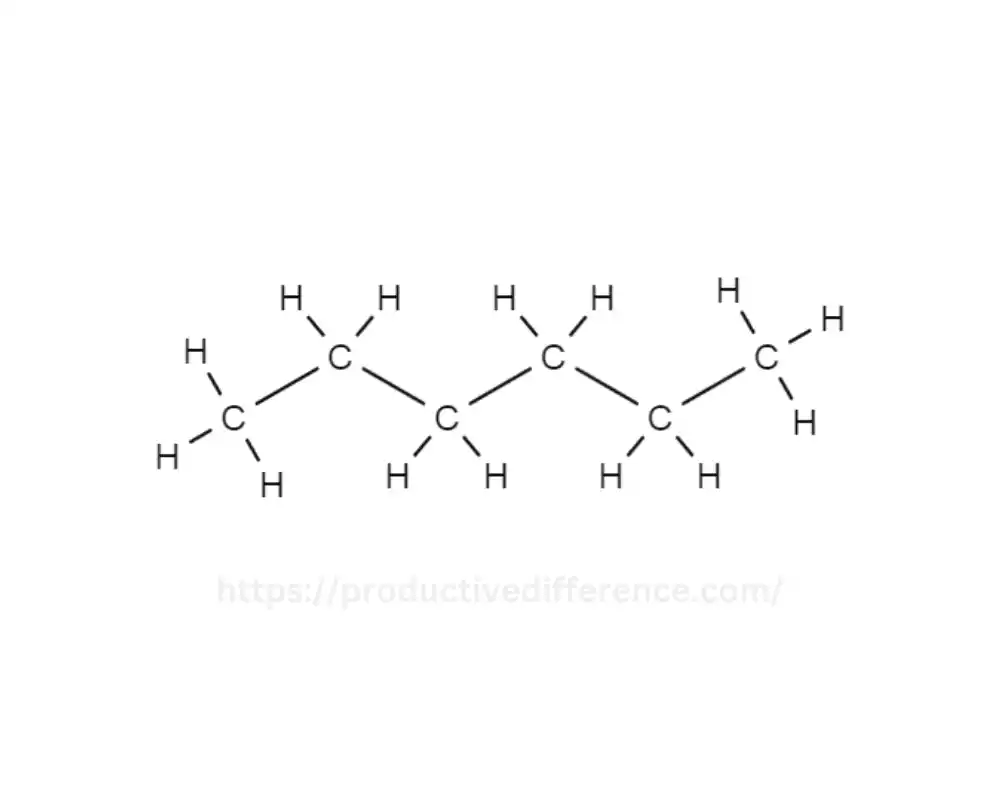
It is impermeable to water, however, it is dissolved with organic solvents. Its nonpolar properties and superior solvent properties make it useful in a wide range of uses, especially when used in laboratories. The n-Hexane solvent is used extensively as a solvent that is non-polar for chemical reaction, and extraction procedures as well as chromatography processes for industrial and research laboratories.
Although n-hexane’s characteristics are extremely beneficial in some applications, however, it’s important to be handled with caution because of the health hazards caused by long-term exposure. Contact with the skin or inhalation of N-hexane vapors can trigger ailments such as discomfort, dizziness and neurological reactions. Thus, safety precautions and adequate ventilation are essential while working with the chemical in order to reduce the risk of exposure.
Difference Between Hexane and n-Hexane
Both are hydrocarbons. This means they’re composed of carbon (C) and hydrogen (H) atoms alone. They are also alkanes. These are which is a form of saturated hydrocarbon. It suggests that all carbon-carbon bonds within their molecular structures are singular bonds.
Hexane:
Chemical Formula: C6H14
Structural Formula: CH3(CH2)4CH3
Explanation: Hexane has a straight-chain structure that has six carbon atoms in one row, each of which is bonded by 2 hydrogen atoms. Its molecular formula C6H14 signifies that it is composed of fourteen hydrogen atoms.
n-Hexane:
Chemical Formula: C6H14
Structural Formula: CH3(CH2)3CH3
The explanation is that n-Hexane can be described as an isomer of the hexane compound, meaning it uses similar chemical formulas, however with an entirely distinct configuration of carbon atoms. In n-hexane, carbon atoms are arranged in an elongated chain that has five carbon atoms per row. The final carbon atom splits off, with three carbon atoms that are bonded to the. This branching is what gives it the designation “n” for normal hexane.
The bottom line is that each hexane and N-hexane has the same molecular formula, C6H14 however their structures differ. Hexane is a linear chain made of six carbon atoms. In contrast, it has a similar chain, however, with one carbon atom that branches off, and then being joined to three carbon atoms. This structural difference results in the different chemical and physical characteristics, which can lead to different applications in different fields.
Physical Properties
Physical properties of hexane as well as the n-hexane
Boiling Point:
- Hexane: the boiling point of hexane can be estimated at 68.7degC (155.7degF). The relatively low temperature of boiling allows it to be used in a variety of industrial processes like extractions using solvents, as well as purification.
- n-Hexane: The boiling temperature of n-hexane may be slightly greater than hexane’s, with about 69.0degC (156.2degF). The tiny variation in boiling temperatures for n-hexane and hexane are because of their identical molecular weights as well as intermolecular forces.
Melting Point:
- Hexane: Hexane has a melting point that is around -95 degrees Celsius (-139degF). Its low melting point lets it remain in liquid state at regular temperature.
- n-Hexane: The n-Hexane compound also has a melt point that is similar that hexane has, which is around 95degC (-139degF).
Density:
- Hexane’s density Hexane is approximately 0.660 g/cm3 when it is heated to 20 degrees Celsius (68degF). It’s more dense than water with 1 g/cm3 of density This property is a contributing factor to its non-compatibility with water.
- The n-Hexane compound has the same density as the hexane compound, which is around 0.660 g/cm3 when heated to 20 degrees Celsius (68degF).
Solubility:
Hexane: Hexane is practically not soluble in water because of its non-polarity. It is however, very solubilized in organic solvents, such as Acetone, ethanol and the benzene.
The same is true for n-hexane. It is almost insoluble in water but is highly easily soluble with organic solvents.
Hexane and n’hexane have identical physical properties due to their close resemblance to molecular structures as well as molecular masses. Their lower boiling temperatures as well as their high volatility and superior solubility when mixed with organic solvents makes them useful in the role of solvents and extract agents for various industrial process.
But their inability to dissolve in water restricts their use in water-based environments. Similar to any other chemical that is used, it is essential to take hexane and n’hexane handling carefully and take the appropriate security measures because of their potential for hazard to health and flammability. dangers that can arise from prolonged exposure.
Production and Sources
Production and sources of Hexane and n-Hexane
Production of Hexane:
- Petroleum Refining: The main source of hexane comes from refining crude oil. In the process of refining it is possible to separate a portion of crude oil referred to as “light naphtha” is obtained that contains hydrocarbons that have the atoms of carbon six. Additional separation and purification methods like distillation is used to remove the naphtha-containing hexane.
- Natural Gas Processing: Hexane is also produced as a byproduct from natural gas production. Natural gas has a variety of hydrocarbons, such as those that have six carbon atoms. They can be separated, and filtered in order to produce Hexane.
Sources of Hexane:
- Industrial applications: Hexane is widely used to dissolve various sectors, with a particular focus on extraction of plant oils, pharmaceuticals as well as the production of adhesives, paints and varnishes.
- Extraction of Oil: Hexane is widely used by the food industry for extraction of oils from nuts and seeds. Extracted oils are often employed in cooking, food processing and many other uses.
- Cleaning Agent: Because of the solvent’s properties as well as its low toxicity, Hexane is utilized as a cleaning agent for certain industrial uses for cleaning equipment, like washing machines and high-precision equipment.
- Production: The production of n-Hexane is a distinct process. is a distinct isomer of Hexane that is made in a similar way to hexane via refinement of natural gas or crude oil processing. In its isomer form, n-hexane shares the same chemical formula (C6H14) like hexane, but it has a different arrangement of carbon molecules and carbon atoms, creating a distinct chemical compound, with distinct characteristics.
The sources of n-Hexane
- Laboratory Use: n-Hexane is found to have substantial use as a solvent in laboratory environments. It is widely used in extraction, purification as well as chromatography procedures within industrial and research labs.
- Organic Synthesis: As a result of its nonpolarity, n-hexane is used as a medium for reaction for organic synthesis. It facilitates the reactions of nonpolar substances.
- Industrial Cleaning: In certain sectors, n-hexane can be used in cleaning, specifically for equipment and machinery, because of its capability to dissolve grease, oils and various organic pollutants.
Application and Uses
Applications and uses for Hexane and n-Hexane
Applications of Hexane:
- Oil Extraction: Hexane is extensively used for its solvent use to extract vegetable oils from seeds as well as nuts like sunflowers, soybeans, and peanuts. It effectively dissolves oil out of seeds and plant materials, which results with high yields of oil and efficient extraction methods that are cost-effective.
- Industrial Solvent: Hexane’s outstanding solvent properties makes it a valuable for a variety of industrial uses. It is widely used an ingredient in the composition of adhesives, glues coatings, paints, and varnishes. It assists in dissolving and dispersing the ingredients of these products to ensure proper application and efficiency.
- Pharmaceuticals: Hexane is a solvent used for pharmaceutical manufacturing processes. It’s used to remove active compounds from herbs and plants or to make a part of pharmaceutical formulations. It aids in the manufacturing of specific medicines.
- Cosmetics: The solvent characteristics of hexane makes it a useful ingredient for the cosmetics industry. It’s used to dissolve and extract natural components utilized in the creation of creams, lotions and makeup products and perfumes.
- Cleaning Agent: Thanks to its toxicity-free and fast rate of evaporation, hexane can be employed as a cleaner across a variety of industries, such as electronic manufacturing and precision equipment production. It is effective in removing oils as well as greases and other pollutants from the surface.
Application of n-Hexane:
Laboratory Solvent: n-Hexane can be widely used in lab situations as a non-polar solvent that can be used for a variety of uses. It is typically used in extraction, purification, as well as process separation in chemical research and research.
Organic Synthesis: Being a non-polar solvent, the n-hexane acts as a medium of reaction to aid in organic syntheses. It aids in the formation of non-polar substances and assists in the creation of certain product.
Industrial Cleaning: Like the chemical hexane, n hexane is employed in a few industries to clean machines and other equipment. Its properties as a solvent enable it to remove the grease, oils and organic residues.
Adhesives and Glues: N-Hexane is used in adhesives as well as glue formulas. It improves the bonding capabilities of these formulations, which ensures solid adhesion to different types of substances.
Processing with Polymers: In certain polymer-related industries, nhexane can be utilized in the process of processing polymers. It aids in the dissolution of and shaping specific types of polymers like polyethylene, to form films, fibers as well as other types of products.
Regulations and Restrictions
Rules and restrictions regarding Hexane and n-Hexane
OSHA Regulations have established permissible levels for airborne chemicals so as to safeguard workers against adverse health impacts. Employers need to be aware of the limitations established and take appropriate protection precautions for employees who work in these chemical substances.
Food and Drug Administration (FDA) Regulations:
The FDA has set guidelines and limitations regarding how much hexane can be used during food processing. It has established maximum residual levels of hexane found in food products in order to guarantee that they are suitable for consumption and will not present health risks for people who consume them.
Globally Harmonized System (GHS) of Classification and Labelling of Chemicals:
The GHS offers a standardized system to classify, label, and identify chemical dangers across the globe. Hexane and n-hexane have particular hazard classifications and containers have to bear suitable GHS labels in order to warn users about the risks they could face.
REACH Regulation (Registration, Evaluation, Authorisation, and Restriction of Chemicals):
Within the European Union, the REACH regulations regulate the registration, assessment, authorization and limitation of chemicals. In this law, n-hexane and hexane could be subjected to limitations or approval for specific uses due to their risk for human health as well as the environment.
National and Regional Regulations:
Many countries and regions might be governed by their own regulations as well as restrictions regarding the use or disposal of n-hexane as well as hexane. They could include restrictions regarding their production and importation, as well as export and use for specific purposes.
Health and Environmental Effects
Health and Environmental Impacts Associated with Hexane and n-Hexane

Health Effects:
- Inhalation: Breathing in the vapors of hexane can cause breathing irritation, headaches nausea, and dizziness. Long-term exposure to high levels of hexane could cause serious effects, such as nerve damage and causing symptoms like numbness muscle weakness, and tingling.
- Contact with the skin: Hexane contact can result in irritation to the skin, as also redness, dryness, and dryness. Prolonged or frequent exposure could lead to dermatitis or skin sensitization.
- Eye Contact: Hexane may cause irritation to the eyes or redness. It can also cause discomfort after contact.
- n-Hexane poisoning: exposure to n-Hexane could cause poisoning with n-hexane which is characterized as a deterioration of your peripheral nervous system. This is a condition called peripheral neuropathy that causes numbness, weakness and pain on the extremities.
- Chronic Exposure: Continuous exposure to n-hexane or hexane may cause chronic negative health effects, with a particular focus for nerves. Long-term exposure may result in peripheral neuropathy which is a chronic disease that may persist after exposure stops.
Environmental Effects:
- Environmental Pollution: Hexane as n-hexane are both VOCs (VOCs) which could lead to pollution of the air.. If they are released into the air they combine with other pollutants and sunlight to create ground-level Ozone which is a major component in air pollution.
- Environmental Pollution: Hexane and n-hexane may not be very water-soluble. However, when spills occur or in the case of improper disposal, they may pollute water sources. The chemicals could remain within the ecosystem, harming aquatic life.
- Solil Contamination: Incorrect removal or accidental spills of the n-hexane and hexane may result in soil contamination which could impact plant growth as well as soil microorganisms.
- Bioaccumulation: A few compounds that are formed through hexane-based breakdown and n’hexane within the ecosystem could possess the capacity to be bioaccumulated within the food chain and pose dangers to the higher levels of trophic level.
- Personal Protective Equipment: Those working with these substances should use the appropriate personal protection equipment such as gloves, goggles and respirators.
- Secure Storage and Handling: Use safe storage and handling procedures to avoid the risk of spills and reduce exposure.
- Disposal: Get rid of these substances according to local guidelines and regulations in order to prevent environmental pollution.
- Substitution: Look into alternatives or less harmful chemicals whenever possible in order to minimize the risk to health and environment.
Conformity with laws, adequate training and risk assessment is essential to protect both the health of humans and the natural surroundings from potential negative impacts of n-hexane as well as hexane.
Comparison Table
Below is a comparison chart highlighting the key differences between Hexane and n-Hexane:
| Attribute | Hexane | n-Hexane |
|---|---|---|
| Chemical Formula | C6H14 | C6H14 |
| Structural Isomer | Straight-chain alkane | Straight-chain alkane |
| Molecular Structure | CH3(CH2)4CH3 | CH3(CH2)3CH3 |
| Boiling Point | Approximately 68.7°C (155.7°F) | Approximately 69.0°C (156.2°F) |
| Melting Point | Approximately -95°C (-139°F) | Approximately -95°C (-139°F) |
| Density | Approximately 0.660 g/cm³ at 20°C | Approximately 0.660 g/cm³ at 20°C |
| Solubility in Water | Practically insoluble | Practically insoluble |
| Main Applications | – Oil extraction | – Laboratory solvent |
| – Industrial solvent | – Organic synthesis | |
| – Pharmaceuticals | – Industrial cleaning | |
| – Cosmetics | – Adhesives and glues | |
| – Cleaning agent | – Polymer processing | |
| Health Effects | – Respiratory irritation | – N-hexane poisoning |
| – Neurological effects with | – Dermatitis | |
| prolonged exposure | – Skin sensitization | |
| Environmental Impact | – VOC contributing to air pollution | – Potential water contamination |
| – Possible soil contamination | – Risk of bioaccumulation | |
| – Environmental persistence in soil | – Potential for soil contamination |
Summary
Hexane, as well as n-hexane, are two hydrocarbons with the same molecular formula C6H14. However, they differ in the structure they use. Hexane is an alkane with a straight chain having six carbon atoms inside the linear chain. it is a isomer of Hexane that has five carbon atoms within a row, and one branching off.
Hexane is extensively used to dissolve various industries, like pharmaceuticals, oil extraction, and cosmetics, and also as a cleaning agent in the industrial sector. It also is utilized for the manufacture of adhesives, glues, paints, and varnishes.