Introduction of Radical and Ion
Radical and Ion are the two most essential elements in the field of chemistry both possessing distinctive properties and behaviors. Radicals, often referred to as free radicals are non-charged particles that have at least one electron that is unpaired within their electron shells’ outermost regions. The unpaired electron is what gives radicals their distinctive significant reactivity because they continuously seek to join with an electron to establish the stable configuration of electrons. Because of their high reactivity radicals participate in an array of chemical reactions that include polymerization, combustion, and the oxidation process.
In contrast, ions are charged particles formed in the event that atoms acquire or lose electrons. If an atom sheds electrons, it transforms into an ion that is positively charged or the cation. In contrast, when an atom is able to gain electrons, it transforms into an ion that is negatively charged or an anion. They play an important role in a variety of chemical reactions since their charged nature allows interaction with other species that are charged, and take part in electrostatic attraction as well as repellents.
A major difference between ions and radicals is in the charge they carry. The charge of radicals is neutral. which means they do not have a charge because the existence of electrons unpaired doesn’t create the overall charge. On the other hand, ions carry the net negative or positive charge, which allows them to interact with particles or polar molecules via electrostatic forces.
Stability is a differentiating feature. The stability of radicals is generally lower than ions because of the fact that they have electrons unpaired which causes an uneven electron structure. The instability of radicals makes them highly responsive, attempting to transfer electrons or to accept them in order for a more steady state. However, ions may be unstable in varying levels according to their configuration in the electronic field and also on their balance of attraction and repelling forces.
Reactivity is the most defining feature of both ions and radicals However, they show various kinds of reactions. Radials are very reactive due to their nonpaired electrons that are able to engage in chemical reactions making or breaking the covalent bonds. Ions on the other, however, show reactivity depending upon their charge and configuration. The positively charged ions, known as cations tend to look for electrons for an electron configuration that is stable, while negatively charged ions also known as anions, are more likely to offer electrons for stability.
Importance of understanding the difference between radicals and ions
Knowing the distinction between ions and radicals is crucial for chemistry research.
Below are some of the main factors that make this knowledge crucial:
Predicting Reactivity: The Radicals and the ions exhibit distinct patterns of reactivity. The radicals, due to their non-paired electrons, have an extremely high level of reactivity and are able to initiate chain reactions as well as radical-based processes. In contrast, the reactivity of ions is contingent on their charge as well as electronic configuration. Understanding the difference the chemists will be able to anticipate the actions of radicals and Ions in the diverse chemical reactions.
Designing Synthesis Strategies: The design of strategies for the synthesis of Ions and radicals is essential to organic synthesis. Knowing their distinct properties will allow scientists to create efficient synthetic pathways and choose suitable reactions. There are many different reactions and processes associated with working with ions and radicals This knowledge assists in the pursuit of desired targets effectively.
The implications for health and the environment: The effects of ions and radicals influence environmental chemistry as well as human health. Radicals, like the radical hydroxyl (*OH) have major roles in the atmospheric reaction and elimination of pollutants. Ions, including metal ions, play a role in the biological process and may affect the health of. Understanding how they behave can assist in dealing with environmental problems and establishing strategies to control pollution as well as health-related uses.
Materials Science and polymer chemistry: Ions and radicals are crucial for the study of polymer science and material chemistry. Radicals play a role in the process of polymerization by free radicals which is a popular method to synthesize polymers. Ions, specifically as Ionic liquids, can be used for a variety of uses in the design of materials as well as energy storage as well as catalysis. Understanding their distinct characteristics allows researchers to modify the properties of materials and enhance performance in a variety of different applications.
Medicinal Chemistry: understanding the role of radicals and ions is vital to medicinal chemists working on the development and discovery of drugs. Radicals, thanks to their chemical reactivity, may be employed in the creation of targeted treatments or even drugs that release specific active substances. Ions, like metal ions, or charged functional groups can be found in pharmaceutical drugs to modify their effects and boost biological activities. The distinction between ions and radicals assists in rationalizing drug development and maximizing the effectiveness of therapeutics.
A fundamental understanding of chemical Systems: The ions and the radicals are essential entities that help to explain the variety and complexity of the chemical world. Understanding the differences between them aids in understanding reaction mechanisms that are triggered by electronic structures as well as the behavior of molecules in general. This allows scientists to improve their knowledge of chemical processes and lay the groundwork for advances in the various areas of chemistry.
Understanding the distinctions between radicals and Ions is essential for understanding the reactivity of compounds, determining methods for synthesis, dealing with issues with health and the environment as well as for advancing materials science, making drug designs more efficient as well as deepening our knowledge of the chemical system. Through understanding the distinct characteristics and behavior of ions and radicals, scientists are able to make educated decisions and come up with innovative strategies that address real-world challenges and accelerate scientific advances.
Definition of radicals
Radicals, sometimes referred to as free radicals are highly reactive chemical species with an unpaired electron or two in their outer electron shell. As opposed to stable molecules or Ions, radicals possess unbalanced electrons because of the existence of electrons that are not paired. Unpaired electrons render radicals extremely reactive as they attempt to pair with a different electron in an atom or molecule so that they can achieve an electron structure that is more stable. Radicals can form through diverse processes like homolytic bond cleavage.
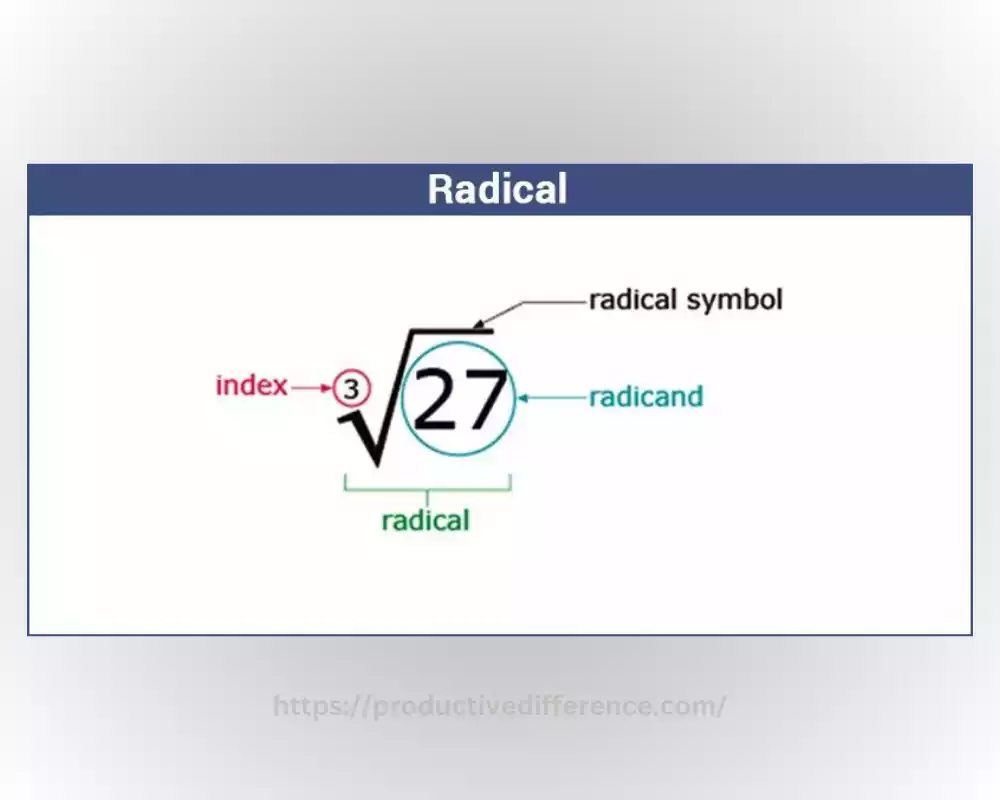
In this process, covalent bonds are broken uniformly, resulting in the creation of two different radicals. Because of their activity, radicals participate in an array of chemical reactions like combustion, oxidation, as well as polymerization. They play an essential part in organic as well as inorganic chemistry. They have important impacts on areas like biochemistry, environmental chemistry as well as material research.
Definition of Ion
Ions are charged electrically which are created as atoms lose or gain electrons. Atoms typically are neutral having an equal amount of positively charged protons inside the nucleus as well as positively charged electrons orbiting within the nucleus. In certain circumstances, there are certain conditions that allow Ionization, which leads to the creation of ions.

When an atom sheds a portion or all electrons through this process, it turns into an ion positively charged, an ion.
This loss of electrons results in an imbalance. It has the presence of more protons than electrons making the cation have the charge of net positive. Cations are characterized by their symbol for elemental with a superscript which indicates how much positive charge. As an example, a sodium ion having one positive charge is depicted by the symbol Na+.
In contrast, when an atom acquires an electron or two it transforms into a negatively charged ion also known as an anion. This gain in electrons causes an increase in negative charge that is characterized by the addition of more protons and electrons which results in the negative charge of the net. Anions can also be represented using their symbol for elemental elements, which is and a superscript indicating the amount of negative charge. A chloride ion that has one negative charge can be depicted by the symbol Cl-.
Ions play an essential part in a variety of chemical reactions and procedures. Since they are charged that attracts ions to opposing charged particles. They also make ionic compounds by electrostatic interactions. They participate in processes like electrolysis which is where the movement of ions within the electrolyte solution causes electroconductive and chemical reactions. Furthermore, ions are essential in the biological system, as they are involved in muscular contractions, nerve conduction as well as a myriad of other biological functions.
Ions are charged electrically by atoms that gain or lose electrons. The net positive charge of ions is a charge, whereas anions carry an inverse charge. Understanding the ion is vital to understanding electrolysis, chemical reactions, and the function in biological processes.
How ions and radicals are formed?
The formation of radicals and ions occurs by a variety of processes.
Ions are formed in various ways:
Ionization: Ionization happens in the event that an atom acquires or loses electrons. This resulted in the creation of Ions. It can occur through processes such as electron transfer in which an atom acquires or loses electrons of another atom or molecules.
Dissociation: Certain substances such as salts may dissociate in water, or other solvents and release ions. Through this process, the substance breaks down into its constituent ions each one carrying a charge.
Acid-Base Reactions: Acid-Base Reactions are characterized by the transfer of protons (H+ Ions) across species. In the event that an acid gives protons, they form an equivalent conjugate base that’s an Ion.
Redox Reactions: Redox (reduction-oxidation) reactions involve the transfer of electrons between species. The species which loses electrons transforms into positively charged ions (cation) and the species that acquires electrons transforms into the negatively charged ion (anion).
Radicals on the other they are formed by processes that lead to breaking covalent bonds as well as the creation of electrons without pairing. Common ways that radicals can be created can be found in:
Homolytic Bond: Cleavage bond cleavage happens in the event that a covalent bond is broken uniformly, with each atom taking one electron out of the bond. The result is the creation of two radicals. Each has an electron that is not paired.
Photolysis: Photolysis is the break of the chemical bond through the absorption of the light energy of photons. The result can be the creation of radicals since the energy derived from the absorption of photons could rupture a bond that is covalent, which can result in the creation the formation of radicals.
Radical Reactions: Radical Reactions also be produced via radical reactions. This is when existing radicals react with molecules, which leads to the exchange of an electron that is not paired as well as the formation of a radical.
It’s crucial to understand that the process of creating radicals and ions is dependent on certain conditions including the presence of appropriate reactants such as energy input or catalysts. The specific mechanisms and paths for ion and radical creation will differ based on the chemical reactions involved as well as the system of operation.
key Differences between Radicals and Ions
The main difference between radicals and ions are as follows:
Charge: Radials are non-charged particles. That is, they possess none of the charge that is net. They don’t acquire or lose electrons and are in a neutral condition. In contrast they are charged particles that have gained the electrons they lost, which results in a net negative or positive charge.
Formation: The formation of radicals is typically created through homolytic bond cleavage which is when the covalent bond is broken uniformly, leading to the formation of unpaired electrons in distinct atoms. Ions on the other, however, can be formed by the heterolytic bond cleavage process or electron transfer. In these processes, electrons either gain or are eliminated, resulting in the creation of charges.
Stability: Generally speaking, radicals are more unstable than ions. The reason for this is that radicals have non-paired electrons that can cause them to be highly reactive and susceptible to participating in chemical reactions. Ions, according to their electronic configuration as well as the proportion of attraction and repelling forces, may have varying levels of stability.
Reactivity: The radicals are extremely reactive because of the presence of electrons unpaired. They are more likely to create or break the covalent bonds leading to chemical reactions. Ions on their own, display the ability to react based on their electron configuration and charge. The positively charged ions (cations) are more likely to search for electrons in order to create an electron structure that is stable as negatively charged anions (anions) tend to be more likely to offer electrons.
Examples and applications Examples and Applications: Radicals play a role in radical reactions as well as processes like Free radical polymerization. They play an important part in a variety of organic reactions and can be found to make organic compounds and polymers. Ions, on the contrary they are utilized across a variety of chemical reactions and procedures.
As a summary Radicals are particles that have no charge and have unpaired electrons. Ions are charged particles formed of the loss or gain of electrons.
Radicals are very sensitive and unstable because of the presence of non-paired electrons. On the other hand, Ions can show varying levels of stability, depending on the configuration of their electrons. Understanding the differences between them is vital to understand their behavior as well as their roles within chemical reactions.
Comparison Chart
Here’s a comparison chart highlighting the key differences between radicals and ions:
| Inspect | Radicals | Ions |
|---|---|---|
| Charge | Uncharged particles (no net charge) | Charged particles (net positive or negative charge) |
| Formation | Formed through homolytic bond cleavage | Formed through heterolytic bond cleavage or electron transfer |
| Stability | Less stable due to unpaired electrons | Stability varies based on electronic configuration |
| Reactivity | Highly reactive due to unpaired electrons | Reactivity based on charge and electronic configuration |
| Examples | Hydroxyl radical (·OH), methyl radical (·CH3) | Sodium ion (Na+), chloride ion (Cl-) |
| Applications | Radical reactions, free radical polymerization | Electrolysis, formation of ionic compounds, biological processes |
Are ions and radicals the same?
Ions and radicals aren’t exactly the same. They are two different types of chemical compounds with different qualities and features.
They are particles charged with charge which are formed when atoms acquire the electrons they lose or gain. They carry a net positive charge or a negative one due to an imbalance in the quantity of electrons and protons. Cations are positively charged particles created by the loss of electrons, whereas anion ions have a negative charge created by the process of electron gain. electrolysis.
However, radicals are charged particles with at least one unpaired electron inside their outer electron shell. This is what makes radicals very reactive since they try to join with an electron in order to create an electron structure that is stable. Radicals play a role in numerous chemical reactions that include combustion, oxidation, as well as polymerization.
Both ions and radicals influence chemical reactions, the charge and electronic structures distinguish their electronic structure and charge. They have the charge of a net, whereas radicals don’t have any charge. In addition, they typically develop due to the loss or gain of electrons. Radicals form via bond cleavage or any different processes that cause the existence of electrons that are not paired.
summary
The ions and the radicals are separate chemical entities with significant differentiators. Radicals are charged particles that have non-paired electrons. This makes them extremely reactive and stable. They form through homolytic bond cleavage. They are engaged in radical reactions and procedures like Free radical polymerization. However, Ions are charged particles that result from the change or loss of electrons. They possess either a negative or net positive charge.
Their stability and reactivity are based on their configuration in the electronic field. Ions form by heterolytic bond cleavage, or electron transfer. They play vital parts in electrolysis, the creation of ionic compounds as well as a variety of biological processes. Understanding the differentiators between ions and radicals are crucial to predicting their behaviors, developing ways to synthesize them, taking care of problems with environmental pollution, improving materials science, improving the design of drugs, and advancing the fundamental knowledge of chemical systems.

