Cation Exchange Capacity and Anion Exchange Capacity are crucial soil properties that determine the soil’s ability to retain and exchange positively charged cations and negatively charged anions, respectively.
Definition of Cation Exchange Capacity
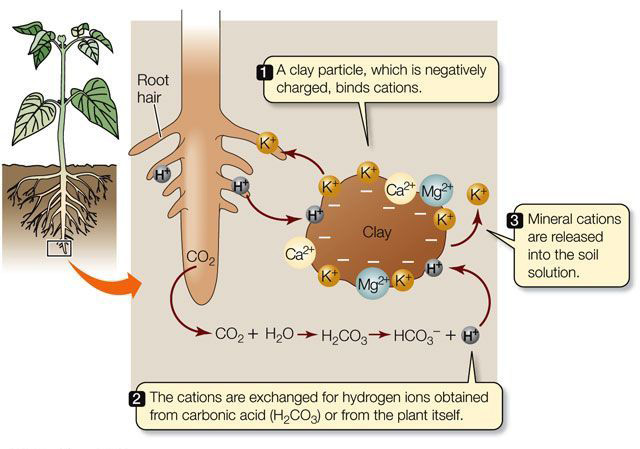
Cation Exchange Capacity (CEC) refers to a soil or other porous material’s ability to retain and exchange positively charged ions known as cations, an essential property that determines fertility and nutrient-holding capacities of soils. CEC measures how many exchangeable cations that a given area is capable of holding including essential elements like potassium (K+), calcium (Ca2+), magnesium (Mg2+) as well as other elements.
CEC can be determined by examining negatively charged sites present on soil particles such as clay minerals and organic matter that attract and hold cations through electrostatic forces, thus making them accessible to plant roots or susceptible to leaching.
Measurement of CEC typically occurs through laboratory analysis. One frequently employed technique involves extracting cations from soil using ammonium acetate solutions and measuring exchanged cations concentration in extracted soil extract samples before reporting CEC as milliequivalents per 100 grams or centimoles of charge per kilogram respectively (meq/100g or cmolc/kg).
CEC plays an instrumental role in soil fertility and nutrient management. Soils with higher CEC have an increased capacity to retain essential plant nutrients by holding more cations; as a result, leaching risks decrease considerably and leaching can be prevented altogether. Being familiar with CEC helps determine appropriate fertilizer application rates as well as make informed decisions regarding amendment strategies to maximize plant growth and agricultural productivity.
Definition of Anion Exchange Capacity
Anion Exchange Capacity (AEC) refers to the ability of soil or porous materials like porous plastic to retain and exchange negatively charged anions – also referred to as anions. Similar to Cation Exchange Capacity (CEC), AEC plays an essential role in soil fertility and nutrient holding capacities, particularly with regard to anions such as nitrate (NO3-), phosphate (PO43-), or sulfate (SO42-).
AEC can be determined by identifying positively charged sites on soil particle surfaces that attract and hold anions with electrostatic forces, permitting their retention and exchange within the matrix of soil. Anions may adsorb onto these positively charged sites before being released back into solution when needed or traded off to other anions within soil solution solutions.
Measuring AEC typically involves laboratory analysis using anion resins or extraction methods designed to quantify exchanged anions. An AEC value can then be expressed either in milliequivalents per 100 grams of soil (meq/100g), or centimoles of charge per kilogram of soil (cmolc/kg).
An effective AEC calculation can play an essential part in understanding nutrient availability, management and environmental impacts. Soils with higher AEC typically retain anions more readily thereby decreasing leaching risk into water bodies as well as impacting plant uptake of anions which impacts growth, overall health and development.
By measuring AEC of soils, farmers and soil scientists can make informed decisions regarding soil management practices such as optimizing fertilizer application rates, selecting suitable amendments and devising ways to limit nutrient losses through leaching. Understanding AEC provides insights that support maintaining soil fertility while safeguarding water quality while supporting sustainable agricultural practices.
Cation Exchange Capacity and Anion Exchange Capacity in soil and water management
Cation Exchange Capacity (CEC) and Anion Exchange Capacity (AEC) play important roles in soil and water management.
Here are their significance and applications in these contexts:
Soil Management:
- Nutrient Availability: CEC and AEC help determine the availability of essential nutrients in soil. CEC determines retention/release cycles of key cations like potassium, calcium and magnesium which are vital to plant development; AEC affects anions like nitrate/phosphate which provide essential plant nutrition. Understanding CEC/AEC helps optimize nutrient management strategies to ensure adequate supplies of essential nutrient uptake by plants.
- Fertilizer Recommendations: CEC and AEC data is utilized to develop fertilizer recommendations tailored specifically for soils with higher CEC or AEC capacities, where high numbers require different approaches than soils with lower numbers; using tailored applications based on this capacity helps maximize nutrient use efficiency while mitigating losses.
- Soil Amendment Strategies: Knowledge of soil CEC and AEC helps in developing effective amendment strategies. For instance, soils with low CEC may benefit from adding organic matter additions to improve their nutrient retention capacity, while those with a low AEC might require amendments which increase anion retention – like adding gypsum for calcium deficiencies.
Water Management:
- Nutrient Leaching: CEC and AEC both play an essential role in managing leaching of nutrients from soil into groundwater or surface waters. Soils with high CEC tend to retain more cations, thus decreasing leaching risk; conversely, soils with lower CEC/AEC have greater propensities for anion leaching leading to lost nutrients as well as potential environmental pollution issues. Understanding these capacities assists managers with controlling leaching risks as well as protecting water bodies from contamination.
- Irrigation Water Quality: AEC plays an essential part in assessing irrigation water. A higher concentration of anions like chloride or bicarbonate could negatively impact soil AEC levels and plant growth; monitoring AEC helps select appropriate sources as well as implement water treatment plans if necessary.
- Soil-Water pH Interactions: CEC and AEC both play key roles in controlling soil and water pH levels, by exchanging cations for anionic concentrations which then influence pH. Knowing these interactions helps managers maintain soil pH for optimum plant nutrition availability and optimal plant development.
CEC and AEC measurements are integral parts of soil and water management practices, serving to inform practices like nutrient management practices, fertilizer recommendations, soil amendment strategies and helping avoid leaching of nutrients into water bodies. Utilizing CEC/AEC data in decision-making processes promotes both sustainable agricultural practices as well as efficient resource administration.
Cation Exchange Capacity (CEC)
Cation Exchange Capacity (CEC) is an essential property of soils which enumerates their ability to retain and exchange positively charged ions – known as cations – thus contributing to fertility, nutrient availability, and overall soil health.
Here are a few important details regarding CEC:
- Definition: CEC (or Exchangeable Cation Exchange Capacity) measures how many exchangeable cations that a soil can hold at once and serves to measure its ability to attract, store and release these essential minerals either for plant uptake or leaching purposes.
- Mechanism: CEC is primarily determined by the presence of negatively charged sites on soil particles, including clay minerals and organic matter. These negatively charged sites attract and hold cations through electrostatic forces, allowing for cation exchange with the soil solution.
- Factors influencing CEC: Several factors affect the CEC of a soil, including soil texture, composition, and pH. Soils with higher clay and organic matter content generally have higher CEC due to their larger surface area and higher number of negative charges. pH also plays a role, as soil acidity or alkalinity can influence the number and activity of exchange sites.
- Measurement and interpretation: CEC is typically measured through laboratory analysis using extraction methods, such as the ammonium acetate method. The extracted cations are quantified, and the CEC is expressed as milliequivalents per 100 grams of soil (meq/100g) or centimoles of charge per kilogram of soil (cmolc/kg). Higher CEC values indicate a greater capacity for cation retention.
- Role in nutrient availability: CEC influences the availability of essential nutrients in the soil. Soils with higher CEC have a greater capacity to retain cations, preventing their leaching and making them more available to plant roots. CEC also affects the nutrient release from soil minerals and organic matter, influencing the long-term nutrient supply to plants.
- Soil fertility and management: Understanding the CEC of a soil is crucial for managing soil fertility and nutrient management. It helps determine appropriate fertilizer application rates, soil amendment strategies, and the overall nutrient-holding capacity of the soil. Balancing cation ratios based on CEC can prevent nutrient imbalances and optimize plant growth.
- Environmental considerations: CEC has implications for nutrient management and environmental sustainability. Soils with low CEC are more prone to nutrient leaching, leading to nutrient losses and potential water pollution. Managing CEC can help minimize environmental impacts by reducing nutrient leaching and maximizing nutrient use efficiency.
Cation Exchange Capacity (CEC) is an essential soil property that plays a pivotal role in nutrient availability, soil fertility and management practices. CEC gives insight into a soil’s ability to retain or release cations – helping agricultural practices as well as sustainable soil management to flourish.
Anion Exchange Capacity (AEC)
Anion Exchange Capacity (AEC) is an essential property of soil that measures their ability to retain and exchange anions – negatively charged ions known as anions. Cation Exchange Capacity (CEC), on the other hand, helps determine availability of nutrients as well as pH regulation and overall soil health.
Here are a few key points about AEC:
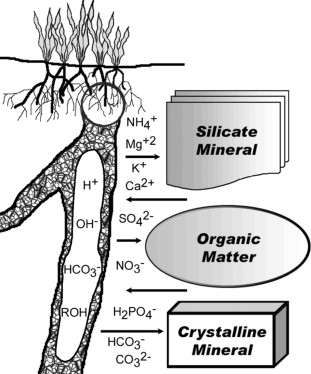
- Definition: AEC is a measure of the total number of exchangeable anions that a soil can hold. It represents the soil’s capacity to attract, retain, and release anions, which affects nutrient availability and the soil’s ability to maintain a desirable pH level.
- Mechanism: AEC is primarily determined by the presence of positively charged sites on soil particles. These positively charged sites attract and hold anions through electrostatic forces, allowing for anion exchange with the soil solution.
- Factors influencing AEC: Several factors can influence the AEC of a soil. Soil properties such as clay mineralogy, organic matter content, and the presence of iron and aluminum oxides can impact AEC. Additionally, soil pH plays a crucial role, as it can influence the availability and concentration of anions in the soil solution.
- Measurement and interpretation: An AEC evaluation can be accomplished through laboratory analysis utilizing anion resins or extraction methods designed to quantify exchanged anions. Once extracted anions have been assessed and quantified, their concentration can then be expressed either in milliequivalents per 100 grams (meq/100g) or centimoles of charge per kilogram of soil (cmolc/kg). Higher AEC values reflect greater anion retention capacities.
- Role in nutrient availability: A soil’s acidity-eluvescence ratio determines its ability to provide essential anions such as nitrate, phosphate and sulfate in sufficient quantity for plant uptake, while also helping prevent leaching risks and leaching risks from taking place. AEC influences both minerals in soil as well as organic matter releases of anions which determine long-term availability to plants.
- Soil pH regulation: AEC contributes to the regulation of soil pH. The presence of exchangeable anions can influence soil acidity or alkalinity. For example, the release of carbonate or bicarbonate anions can buffer soil pH towards alkalinity, while the presence of sulfate or nitrate anions can contribute to soil acidification.
- Soil amendment strategies: Understanding the AEC of a soil helps in selecting appropriate soil amendment strategies. Soils with low AEC may require amendments that enhance anion retention, such as applying gypsum to address calcium deficiencies or incorporating organic matter to improve nutrient holding capacity.
- Environmental considerations: AEC plays an essential role in mitigating both nutrient losses and environmental impacts, especially soils with lower AEC that are more prone to anion leaching that causes losses of vital nutrients that end up polluting our waterways. Thus managing AEC effectively can minimize leaching while guaranteeing efficient utilization while simultaneously safeguarding water quality.
Anion Exchange Capacity (AEC) is an essential soil property, impacting everything from nutrient availability, soil pH regulation and environmental sustainability to guiding nutrient management practices and sustainable soil management strategies. AEC measures soil’s ability to retain or release anions that support efficient management practices as well as sustainable soil strategies.
Comparison between Cation Exchange Capacity and Anion Exchange Capacity
Cation Exchange Capacity and Anion Exchange Capacity are two important properties of soils that involve the exchange of charged ions.
Here is a comparison between Cation Exchange Capacity and Anion Exchange Capacity:
- Definition and Concept:
- Cation Exchange Capacity : CEC is the ability of a soil to retain and exchange positively charged ions, known as cations.
- Anion Exchange Capacity: AEC is the ability of a soil to retain and exchange negatively charged ions, known as anions.
- Ion Types:
- Cation Exchange Capacity : CEC deals with the exchange of cations, such as potassium (K+), calcium (Ca2+), magnesium (Mg2+), etc.
- Anion Exchange Capacity: AEC involves the exchange of anions, such as nitrate (NO3-), phosphate (PO43-), sulfate (SO42-), etc.
- Exchange Mechanism:
- Cation Exchange Capacity : Cations are attracted to and retained by negatively charged sites on soil particles through electrostatic forces.
- Anion Exchange Capacity: Anions are attracted to and retained by positively charged sites on soil particles through electrostatic forces.
- Influence on Nutrient Availability:
- Cation Exchange Capacity : CEC affects the availability of cations in the soil, influencing their retention, release, and uptake by plants.
- Anion Exchange Capacity: AEC affects the availability of anions in the soil, influencing their retention, release, and uptake by plants.
- Factors Affecting Capacity:
- Cation Exchange Capacity : Factors influencing CEC include soil texture, composition, organic matter content, clay mineralogy, and soil pH.
- Anion Exchange Capacity: Factors influencing AEC include soil texture, composition, organic matter content, presence of iron and aluminum oxides, and soil pH.
- Measurement:
- Cation Exchange Capacity : CEC is typically measured through laboratory methods involving extraction with solutions like ammonium acetate, and the concentration of exchanged cations is determined.
- Anion Exchange Capacity: AEC is measured through specific anion resins or extraction methods designed to quantify the concentration of exchanged anions.
- Role in pH Regulation:
- Cation Exchange Capacity : CEC can influence soil pH by affecting the exchange of cations and their impact on soil acidity or alkalinity.
- Anion Exchange Capacity: AEC can influence soil pH through the exchange of anions and their impact on soil acidity or alkalinity.
- Environmental Considerations:
- Cation Exchange Capacity : CEC is related to nutrient retention and can help prevent nutrient leaching, reducing the risk of water pollution.
- AEAnion Exchange CapacityC: AEC is related to anion retention and can help minimize anion leaching, reducing the risk of nutrient losses and water pollution.
Cation Exchange Capacity and Anion Exchange Capacity represent the soil’s ability to exchange cations and anions. Both play key roles in terms of nutrient availability, pH regulation and environmental impacts; understanding both factors is integral for optimizing fertility management practices as well as responsible agriculture practices.
Comparison chart
| Topics | Cation Exchange Capacity (CEC) | Anion Exchange Capacity (AEC) |
|---|---|---|
| Definition | Ability to retain and exchange cations | Ability to retain and exchange anions |
| Ion Types | Cations (e.g., K+, Ca2+, Mg2+) | Anions (e.g., NO3-, PO43-, SO42-) |
| Exchange Mechanism | Attracted to negatively charged sites | Attracted to positively charged sites |
| Influence on Nutrient Availability | Affects availability of cations | Affects availability of anions |
| Factors Affecting Capacity | Soil texture, composition, organic matter, clay mineralogy, soil pH | Soil texture, composition, organic matter, presence of iron and aluminum oxides, soil pH |
| Measurement | Extraction methods with cation-specific solutions, quantification of exchanged cations | Anion-specific resins or extraction methods, quantification of exchanged anions |
| Role in pH Regulation | Can influence soil pH through cation exchange | Can influence soil pH through anion exchange |
| Environmental Considerations | Helps prevent nutrient leaching and water pollution | Helps minimize anion leaching and nutrient losses |
Conclusion
Cation Exchange Capacity and Anion Exchange Capacity are vital properties of soil that measure retention and exchange of charged ions – CEC refers to positively charged cations while Anion Exchange Capacity involves negatively charged anions – within soils, in particular. Both properties play an essential role in terms of nutrient availability, pH regulation and environmental impacts.
Cation Exchange Capacity measures soil’s ability to retain and exchange cations, impacting both its fertility and availability of nutrients. Cation Exchange Capacity measurements help with fertilizer application rates management as well as optimizing plant growth through controlling nutrients availability and managing availability. Its measurements depend on factors like texture composition organic matter content pH. Cation Exchange Capacity measurement are beneficial in helping determine appropriate rates.