Peroxide and Dioxide
Peroxide and Dioxide could be described as two chemicals that differ in their characteristics as well as composition. Peroxide is an expression used to refer to the chemical substance that contains the isotope peroxide (O2^2+) also known as an oxygen-oxygen bond. This is often referred to the hydrogen peroxide (H2O2) it is comprised of two hydrogen molecules that are joined with oxygen molecules. Peroxides are known for their oxidizing properties and are able to let oxygen out when they break up.
But it’s an element that has the presence of two oxygen molecules. Dioxides are identified through their functions in a wide range of chemical reactions and their impact in the process of environmental degradation.
The most significant difference between dioxide and peroxide is in their structures as well as the level of reactivity. Peroxides are generally more volatile and more prone to break down as well as release oxygen. They are commonly used to bleach as well as disinfectants and are utilized in numerous industrial processes. Dioxides, however, on the other hand, are much more stable chemicals and are frequently used to perform important tasks in the areas of carbon dioxide in the climate, and also as air pollution.
Understanding the distinctions between peroxide and dioxide are crucial for correct management, security and their use across different sectors.
What is the history of peroxide and Dioxide?
The development of peroxides as well as dioxides is interwoven in the study and discovery of oxygen and its components.
This is a quick overview of their past:
Peroxide:
- It was in 1818 that Louis Jacques Thenard, the French chemical chemist, discovered hydrogen peroxide (H2O2) through the reaction of barium peroxide in nitric acids.
- Thenard’s research caught the attention of chemical scientists, and the subsequent study led to the identification of additional peroxides.
- In the late 19th century, researchers continued to research peroxide compounds as well as their effects.
- Hydrogen peroxide gained recognition for its antiseptic properties and used for disinfection during World War I.
- In time, the applications have expanded into bleaching agents propellants for rockets, as well as industrial process.
Dioxide:
- The story of the history of dioxides is inextricably linked to the research and development of elements that are chemical.
- Carbon dioxide (CO2) is in use since antiquity because it’s naturally produced by combustion and respiration.
- In the 18th century Joseph Black, a Scottish scientist, carried out experiments with carbon dioxide. He also recognized its importance in the notion of “fixed air.”
- The scientific understanding of the sulfur dioxide (SO2) and the properties it has developed between the 19th and 18th century especially in connection with the chemical industry, as well as the environmental impacts.
The overall history of the peroxides and dioxides is an ongoing process of discovery, separation and understanding of these substances along with their properties as well as their numerous applications across a variety of fields. Research and developments continue to increase our understanding of both peroxides and dioxides, as well as their role in the contemporary world.
Definition of peroxide
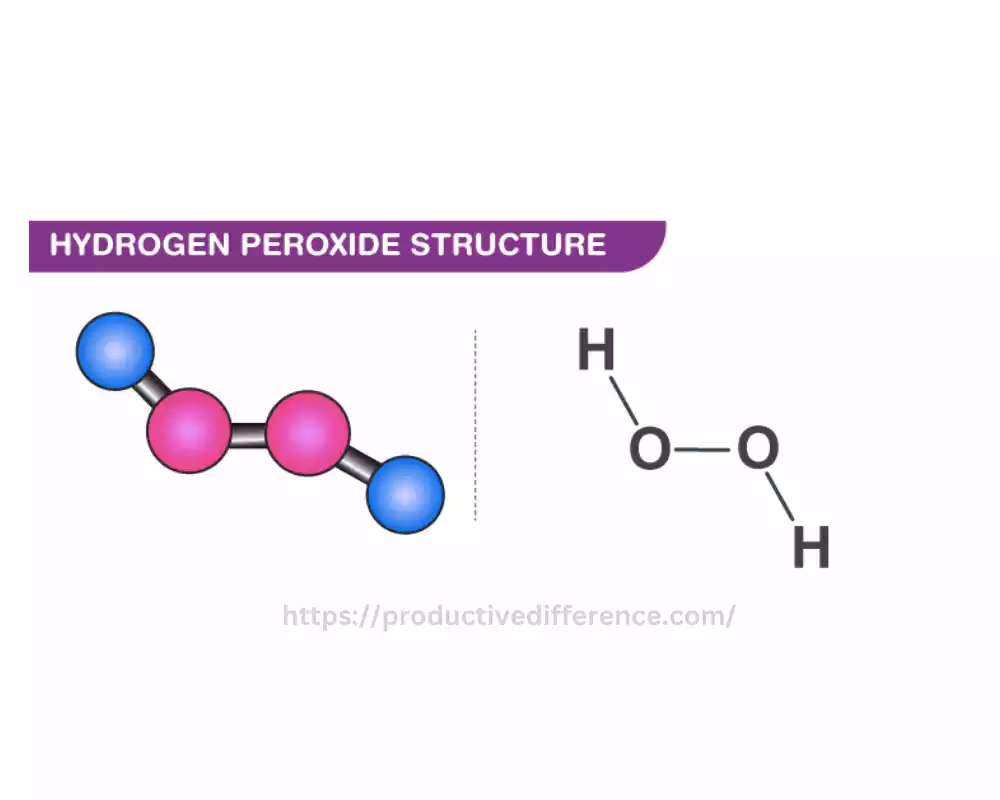
Peroxides are chemical compound that consists of the peroxide isotope (O2^2+) or comprises one oxygen-oxygen bond. Peroxides are distinguished by their presence of the group of peroxide (-O-O-) in their molecular structures. The most renowned peroxide examples is the hydrogen-peroxide (H2O2) comprised by two hydrogen molecules, which bond by oxygen molecules.
Peroxides are well-known for their properties of oxidation that means they have the capability to assist the occurrence of an oxidation reaction. They release oxygen as they are decomposed, which is why they can be useful in the form of oxygen for various purposes. Hydrogen peroxide for example is used extensively to treat bacterial infections, disinfectants bleaching agent, as well as an the oxidizer used in industry.
It is crucial to remember that, while peroxides are a important properties, they could be unsteady and dangerous if handled incorrectly. It is important to take the appropriate precautions when handling peroxides to protect yourself.
Definition of Dioxide
Dioxides can be defined as chemical compounds consisting of two oxygen atoms connected by bonds to elements or. Their name refers to their presence, with different shapes depending on which element or element compounds they’re associated with.
Composed of carbon atoms bonded together with oxygen molecules, carbon dioxide is naturally present within Earth’s atmosphere and produced via combustion, respiration or industrial processes.

Silica dioxide (SiO2) – commonly referred to as silica – also falls within this group and each has unique qualities depending upon their chemical attributes and uses.
Dioxides have an enormously important effect on our lives and environment. Carbon dioxide plays a pivotal role in climate change due to its ability to retain temperatures within air molecules and contribute to greenhouse effects; nitrogen dioxide and sulfur dioxide emissions also have serious ramifications on peoples health as well as on environmental degradation.
Understanding the characteristics and behavior of dioxides are vital in fields like atmospheric science, environmental studies and pollution control – not to mention industrial processes and pollution prevention.
Key Differences between Peroxide and Dioxide
There are many key differences in the peroxide compounds and those of dioxide. We’ll look at them in detail:
Composition and Structure:
- Peroxide: Peroxides are composed of the ion of peroxide (O2^2+) or contain an oxygen-oxygen bond (-O-O–) in their molecular structures. Peroxide of Hydrogen (H2O2) is an famous example of a compound.
- Dioxide: Dioxides are composed of two oxygen atoms that are bonded to an element or elements.
Oxygen Content:
- Peroxide: Peroxides usually have more oxygen in comparison to dioxides. As an example, hydrogen peroxide (H2O2) comprises two oxygen atoms in a molecule.
- Dioxide: Dioxides are composed of two oxygen atoms in a molecules. Carbon dioxide (CO2) contains two oxygen atoms that are bonded to carbon atoms.
Reactivity and Stability:
- Peroxide: Peroxides generally more volatile and unstable than dioxides. They readily undergo decomposition and release oxygen, frequently exhibiting oxidizing characteristics.
- Dioxide: Dioxides tend to be more robust and stable in comparison to peroxides. They’re generally chemically inert, and have different characteristics based on the element they bond with.
Toxicity and Safety Considerations:
- Peroxide: Certain peroxides, like hydrogen peroxide may be harmful or corrosive when present in large quantities. Safe handling practices and precautions are essential to avoid incidents or adverse consequences.
- Dioxide: Dioxides like carbon dioxide, are typically not harmful to human beings.
Applications:
- Peroxide: Peroxides are used in a wide range of areas like health care (antiseptics and disinfectants) and bleaching agents propellants for rockets, as well as industrial processes that require the use of oxidizing agents.
- Dioxide: Dioxides come with a variety of applications too. As an example, carbon dioxide can be used to carbonate drinks, fire extinguishers and even as refrigerant. Sulfur dioxide can be used in the chemical synthesis process and also for preservation of food items.
The understanding of the fundamental differences in the peroxide and dioxide compound is vital for correct handling, safe considerations as well as the particular applications they can be used across various fields and industries.
Comparison Chart
Here’s a comparison chart highlighting the key differences between peroxide and dioxide compounds:
| Inspect | Peroxide | Dioxide |
|---|---|---|
| Composition and Structure | Contains the peroxide ion (O2^2-) or oxygen-oxygen single bond (-O-O-) within their molecular structure | Consists of two oxygen atoms bonded to another element or elements |
| Oxygen Content | Typically higher oxygen content | Contains two oxygen atoms per molecule |
| Reactivity and Stability | More reactive and less stable | More stable and less reactive |
| Toxicity and Safety Considerations | Some peroxides can be toxic or corrosive in high concentrations | Generally non-toxic, but high concentrations or specific environments can pose asphyxiation risks |
| Applications | Used as antiseptics, disinfectants, bleaching agents, rocket propellants, and oxidizing agents in various industries | Utilized in carbonation of beverages, fire extinguishers, refrigeration, chemical synthesis, and food preservation |
| Examples | Hydrogen peroxide (H2O2) | Carbon dioxide (CO2), sulfur dioxide (SO2) |
| Environmental Impact | Can release oxygen upon decomposition | Carbon dioxide contributes to climate change; sulfur dioxide contributes to air pollution |
| Stability | Less stable and prone to decomposition | More stable and less prone to decomposition |
Safety Precautions and Handling
If you are working with peroxides as well as dioxides, it’s essential that you follow the safety guidelines to reduce risks and guarantee the safety of handling.
Here are some guidelines for safety:
Protect yourself with Personal Protective Equipment (PPE): Put on the appropriate PPE like gloves, safety glasses or lab coats and even respiratory protection when needed for protection from chemical exposure.
Ventilation: Be sure to work in a ventilated location or with fume hoods to avoid the build-up of dangerous gases or vapors.
Storage: Place peroxides as well as dioxides in the correct cabinets and containers, free of incompatible substances. Be sure to follow the manufacturer’s guidelines on storage temperature and conditions.
Mixing and handling: Avoid contact with the eyes or skin. Be careful when handling these substances to avoid spills or release. Beware of mixing substances that are not compatible, because it could cause hazardous reactions.
Disposal: Remove dioxides and peroxides according to the local guidelines and regulations. Avoid putting the waste in normal trash, or pour them into drains with out the proper disposal.
Decomposition and Stability: Take note of the decomposition potential and stability of peroxides. Do not expose the peroxides to extreme heat, shock or friction since they can cause explosive decomposition, or even explode.
Safety and Preparedness for Emergencies: Get familiar about emergency protocols, which include the evacuation route, spill control and handling accidents. Be sure to keep the proper security equipment, including eyewash stations and spill kits easily accessible.
Knowledge and Training: Make sure that those who work in the field of peroxides or dioxides are given the proper education on hazards as well as handling protocols and the protocols for responding to emergencies.
It is crucial to remember the fact that safety measures may differ based on the specific dioxide or peroxide compounds that is being used. Always read the safety data sheets (SDS) and adhere to the instructions that are provided by the manufacturer or other authorities that are relevant to the specific substance you’re working with.
Environmental Impact
Peroxides and dioxides both pose distinct environmental concerns that must be carefully considered. Peroxides release oxygen upon degradation, potentially disrupting ecosystems and aquatic environments if excessive quantities are released into the environment. Furthermore, improper disposal can result in water contamination, negatively affecting aquatic life. Furthermore, certain peroxides at higher concentrations or industrial effluents may contain toxic concentrations which necessitate proper handling and disposal to safeguard environmental safety.
To counter these environmental impacts, various strategies can be employed. Carbon capture and storage (CCS) technologies aim to capture CO2 emissions from industrial processes and store it underground rather than release into the atmosphere; switching to renewable energy sources helps decrease fossil fuel reliance while simultaneously decreasing emissions; stricter regulations as well as pollution control technologies may help lower sulfur dioxide, nitrogen dioxide emissions or any other pollution forms; while proper disposal/recycling practices for peroxides/chemicals is imperative in protecting the environment from further contamination.
Understanding the environmental effects of peroxides and dioxides is paramount to taking measures that minimize emissions, ensure responsible handling and disposal, mitigate effects on ecosystems, air quality and climate change, while creating sustainable practices and environmental awareness to lessen negative consequences and ensure we move towards a greener planet.
What are the advantages and disadvantages of Peroxide and Dioxide
Advantages of Peroxide:
Oxidizing Properties: Peroxides are oxidizing substances that have excellent properties. They are thus used in a broad variety of uses, including bleaching, disinfectants and other purposes as well as oxidizers, which are employed in industrial process.
Versatility: Peroxides have a wide range of applications. that they are able to be used in a myriad of ways for cleaning, sterilization and bleaching hair, as well in wastewater treatment, and treatment of pollution in the environment.
Antiseptic Property: Hydrogen Peroxide specifically is widely known as an antiseptic. It is frequently used in cleansing of wounds and in the disinfection.
Decomposition: Peroxides may be broken down and released oxygen. This can be beneficial in specific situations where oxygen flow is essential.
Disadvantages of Peroxide:
Storage and Stability: Certain peroxides have a tendency to be unstable and are prone to decomposition in certain conditions, making the storage and handling of these substances difficult.
Reactivity: Due their nature of oxidizing peroxides are able to react with other materials and could trigger explosions or other hazardous reactions if they are they are mixed with non-compatible materials.
Toxicity: Certain peroxides especially in large quantities are corrosive and harmful, and pose dangers to health of humans and the surroundings. It is important to handle them carefully and removed safely.
Summary
Dioxides can be defined as chemical compounds consisting of two oxygen atoms connected by bonds to elements. Their name refers to their presence, with different shapes depending on which element or element compounds they’re associated with. Composed of carbon atoms bonded together with oxygen molecules, carbon dioxide is naturally present within Earth’s atmosphere and produced via combustion, respiration or industrial processes.