Oxygen and carbon dioxide are two fundamental gases crucial for life on Earth. While both play essential roles in different processes, they exhibit distinct properties and behaviors. In this article, we will explore the key differences between oxygen and carbon dioxide, delving into their chemical makeup, functions, and impacts on the environment and human health. By the end, you’ll gain a deeper understanding of these gases and their significance in the natural world.
Why it is important to know the difference between Oxygen (O2) and Carbon Dioxide
The main difference is between carbon dioxide and oxygen. Oxygen is a diatomic molecule with two oxygen molecules, whereas carbon dioxide has one carbon atom plus two oxygen atoms.
Carbon dioxide and oxygen gases play a crucial role in Earth’s atmospheric composition. It is because it’s important for all living things. We also need oxygen to breathe, which produces carbon dioxide during respiration. This oxygen is needed to create energy (ATP), inside the cells of living organisms, through a process called cellular respiration.
Plants use carbon dioxide to create carbohydrates through photosynthesis. Plants are involved in maintaining oxygen and carbon dioxide levels in the air.
What is Oxygen?
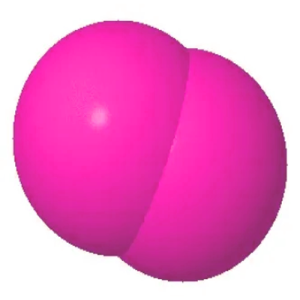
The diatomic oxygen gas is composed of two oxygen atoms that are bonded together via a double bond. The two oxygen atoms are bound together by covalent chemical bonds. The oxygen molecule, therefore, is a covalent or molecular complex. This compound is in a gaseous form at standard temperatures and pressures.
Moreover, about 21% is found in our atmosphere. It is also odorless and colorless. This gas is essential for the life of Earth, as we use it for cell respiration. Oxygen atoms also play a vital role in the biological system, including carbohydrates, proteins, and nucleic acid.
The photosynthesis process is important, in that plants utilize the sunlight’s energy to create carbohydrates and oxygen out of water and carbon dioxide. Ozone is an allotrope that forms a protective layer of air in the upper atmosphere.
This gas has several positive characteristics. It dissolves easily in water and can be transported throughout the body by body fluids. We can also obtain oxygen gas of high purity by fractional distillation from liquefied air. It reacts with every element to produce oxides, except for inert gasses. It is therefore a very good oxidizing gas. The combustion of fuels is also dependent on oxygen. Oxygen can be used in welding and hospitals as well as many other industries.
What is Carbon Dioxide (CO2)?
The carbon dioxide molecule is triatomic, consisting of one oxygen and two carbon atoms. The double bonds between each oxygen atom and carbon are formed by the oxygen atoms. The molecule is linear in shape. Carbon dioxide has a molecular mass of 44 g -1. It is colorless and has the chemical formula CO2. Carbonic acid is formed when water dissolves. This gas is also denser. Carbon dioxide concentration in the air is only 0.03%.
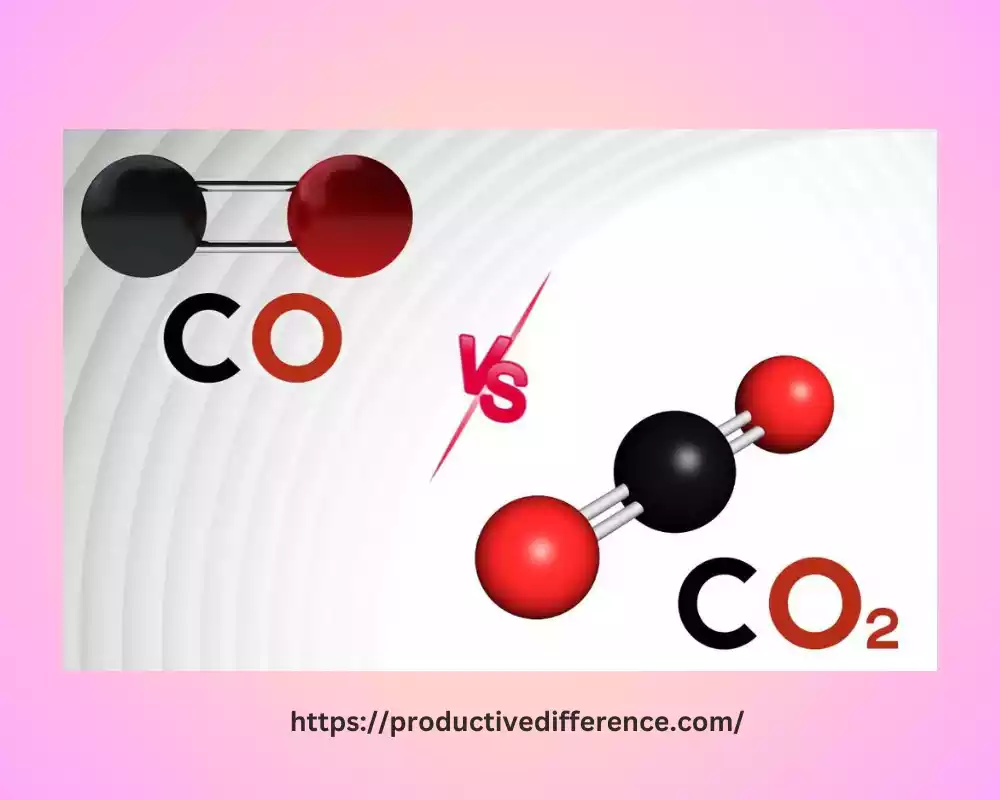
Figure 02: Triatomic Molecule of Carbon Dioxide
The carbon cycle balances the amount of carbon dioxide in the air. This gas is released into the air by a variety of natural processes, including respiration and volcano eruptions, as well as human activity, such as burning fossil fuels in factories and vehicles. The process of photosynthesis also removes the carbon dioxide in the atmosphere, which deposits the carbonates over time.
The carbon cycle is disrupted by human interference, such as fossil fuel combustion and deforestation, which increases the level of CO2. Acid rain, global warming, the greenhouse gas effect, and other environmental issues have been caused by this. It is used to produce soft drinks in the baking industry and as a fire extinguisher.
What is the difference between oxygen and carbon dioxide?
- Oxygen is represented by the chemical formula O2, whereas carbon dioxide is shown as CO2.
- Carbon dioxide and oxygen play different roles in respiration. Inhaling oxygen and exhaling carbon dioxide is the way we breathe.
- The molecular structure of CO2 and O2 is different. Carbon dioxide is made up of two oxygen atoms, while oxygen consists of two molecules.
- The mass of CO2 is slightly greater than that of O2. While oxygen has a molecular mass of 32 grams, CO2’s molecular weight is 44 grams. The gases in the air do not layer up because CO2 is heavier. The various gases in the atmosphere are kept mixed by convection and diffused.
- O2 is necessary for combustion. When a fuel reacts and emits heat, it is called combustion or burning. This reaction is started by a small heat burst or spark. The combustion process cannot take place if oxygen is absent. In contrast, CO2 does not burn and is non-flammable. By starving the fire of O2, CO2 will extinguish a flame.
- Oxygen boils and freezes at about -183° Celsius. Carbon dioxide boils and freezes at just -57.6 degrees Celsius.
Human Health Impact
The difference between oxygen and carbon dioxide is not only essential in understanding atmospheric processes but also in comprehending how these gases affect human health. Both oxygen and carbon dioxide play crucial roles in maintaining human life, and their levels must be within a specific range for optimal well-being.
- Oxygen Deficiency and Hypoxia Oxygen is vital for cellular respiration, a process through which our cells produce energy. When the body does not receive sufficient oxygen, a condition known as hypoxia occurs. Hypoxia can be caused by various factors, such as high altitudes, respiratory diseases, or exposure to hazardous gases. Severe cases of hypoxia can lead to dizziness, confusion, shortness of breath, and even unconsciousness. Immediate medical attention is necessary in such situations.
- Carbon Dioxide Toxicity While oxygen is essential for life, excessively high levels of carbon dioxide can be harmful to human health. Carbon dioxide toxicity, also known as hypercapnia, occurs when there is an excessive buildup of CO2 in the body. Hypercapnia can result from inadequate ventilation in enclosed spaces, exposure to certain chemicals, or respiratory conditions that hinder proper CO2 elimination. Symptoms of carbon dioxide toxicity include headaches, dizziness, confusion, and rapid breathing. In severe cases, it can lead to respiratory failure and unconsciousness.
- Indoor Air Quality The indoor air quality in our homes and workplaces is essential for maintaining good health. Proper ventilation and air exchange are crucial to ensure a balance between oxygen and carbon dioxide levels. Poor indoor air quality can lead to fatigue, headaches, and respiratory problems. To improve indoor air quality, it’s essential to ensure adequate ventilation, avoid exposure to indoor pollutants, and keep indoor plants, which can help in the natural exchange of gases.
- Oxygen Therapy Oxygen therapy is a medical intervention used to provide supplemental oxygen to individuals with respiratory conditions or those experiencing hypoxia. It is commonly used for patients with chronic obstructive pulmonary disease (COPD), pneumonia, or other lung-related issues. Oxygen therapy can significantly improve the quality of life and help patients breathe more easily.
- Carbon Dioxide Monitoring in the Workplace In certain work environments, such as laboratories or industrial settings, there may be a risk of elevated carbon dioxide levels due to specific processes or insufficient ventilation. Employers must monitor CO2 levels regularly to ensure a safe working environment for their employees. Proper safety protocols should be implemented to address any potential issues.
- Carbon Dioxide and Mental Function Some studies suggest that high levels of carbon dioxide in indoor environments can affect cognitive function and decision-making. This is particularly relevant in office settings, where productivity and focus can be influenced by air quality.
- Air Pollution and Respiratory Health While carbon dioxide itself is not considered a primary air pollutant, activities that release CO2 often coincide with other harmful emissions, such as particulate matter and nitrogen oxides. Prolonged exposure to air pollution can have adverse effects on respiratory health, exacerbating conditions like asthma and chronic bronchitis.
- Climate Change and Public Health The increased concentration of carbon dioxide in the atmosphere contributes to climate change, which has wide-ranging implications for public health. Rising temperatures can lead to more frequent and severe heatwaves, affecting vulnerable populations, such as the elderly and children. Additionally, climate change can alter disease patterns, affect food and water security, and lead to the spread of certain infectious diseases.
The difference between oxygen and carbon dioxide extends beyond their roles in the atmosphere. Understanding how these gases impact human health is crucial for addressing environmental challenges and promoting well-being. Proper ventilation, regular monitoring, and sustainable practices are key to maintaining a healthy balance of these gases in our living spaces and ensuring a sustainable future for generations to come.
Side-by-Side Comparison – Oxygen vs Carbon Dioxide
Oxygen |
Carbon dioxide |
| Gases that are present in the air at a concentration of 21% or less, including oxygen, is an essential gas. | Less than 1% of the Earth’s surface is covered by carbon dioxide. The concentration is near 0.03%. |
| Most animals inhale oxygen. | Most animals exhale carbon dioxide. |
| The oxygen gas is diatomic, with 2 oxygen atoms bound together by a double bond. | Triatomic carbon dioxide molecules are found in nature. The carbon dioxide molecule is composed of one oxygen and two carbon atoms that are covalently bound together. |
| This is an element of modern periodicity that belongs to group 16. | This is not an element. It’s a molecule. |
| Oxygen has an atomic number of 8. Oxygen’s chemical formula is O2. Oxygen is represented by the symbol O. Oxygen has a molar weight of 16 grams per mol. | Carbon dioxide has the chemical formula CO2. Carbon dioxide has a mass molar of 44g/mol. |
| Other industries use it for hospitals, welding materials, etc. To clean dirty water, pure oxygen is pumped into the system. | In the production of cold drinks and soft drinks, carbon dioxide is also used. Carbon dioxide is used also in the baking industry. |
| As the oxygen levels are low at high altitudes, athletes need oxygen cylinders to reach high altitudes. | The fire extinguisher uses carbon dioxide gas. |
Exchange of Oxygen with Carbon Dioxide
Inhaling oxygen is the primary purpose of our respiratory system. The oxygen that is inhaled enters the lungs and then reaches the Alveoli. Each layer of alveoli cells and surrounding capillaries is only 1 cell thick, and they are very close in contact. The thickness of this barrier between blood and air is approximately 1 micron. This air-blood membrane allows oxygen to quickly pass into the blood through the capillaries. Carbon dioxide is also exhaled from the alveoli after it passes through the air-blood barrier.
The lungs send oxygenated blood through the pulmonary venous system to the left of the heart. This side pumps blood throughout the body. (See Function of the Heart). The superior and inferior vena vegas return oxygen-deficient blood rich in carbon dioxide to the left side of the body. After the blood has been pumped to the lungs through the pulmonary arterial, it is oxygenated and releases carbon dioxide.
Even when the body is resting, approximately 5 to 8 liters (1 to 2 gallons), of air are drawn into and out of the lung each minute. About three-tenths a gallon (about 3 tenths a quart), of oxygen, is also transferred to the blood.
A similar amount of carbon dioxide is also exhaled as it moves from the alveoli to the blood. An individual can exhale and inhale more than 100 liters of air (approximately 26 gallons of air) per minute, and then extract three liters of oxygen (a bit less than one gallon of oxygen) from the air. It is possible to measure the energy expenditure of the body by measuring the oxygen consumption rate. Respiratory muscles are responsible for breathing in and out.
The transfer of oxygen to blood through the lungs is dependent on three processes: diffusion, perfusion, and ventilation.
- The process of ventilation is when air enters and leaves the lungs.
- The body does not exert any effort or energy to diffuse gases between alveoli in the lungs and capillaries.
- The cardiovascular system circulates blood through the lungs by perfusion.
Circulation is a vital link that connects the oxygen-rich atmosphere to the oxygen-consuming cells in the body. The ability to deliver oxygen to muscle cells in the entire body is dependent not just on the lungs, but also the ability to transport oxygen through the blood and the circulatory system. A small portion of blood that is pumped by the heart enters bronchial vessels and feeds the airways.
Conclusion
oxygen and carbon dioxide are two gases that play crucial roles in supporting life on Earth. While oxygen is essential for respiration and combustion, carbon dioxide is vital for photosynthesis and regulating the Earth’s temperature. Understanding the differences between these gases is essential for promoting environmental sustainability and ensuring a habitable planet for future generations.