Isotopes and Isobars
The isobars and the isotopes are both fundamental concepts within the area of chemistry and nuclear physics which describe the variations that occur in nuclear nuclei.These causes variations in their atomic mass and chemical properties, while still being able to be maintained. Carbon-12, carbon-13 and carbon-14 are isotopes of carbon. They each have six protons, but different number of neutrons. The isotopes are unstable or radioactive. Moreover, can be used for a wide range of disciplines like radiocarbon dating nuclear medicine and environmental research.
However isobars can refer to various chemical elements, each with the same mass which represents the total number of neutrons and protons inside their nuclei. Although they have different atomic numbers isobars have similar mass and consequently, similar energies. The elements could have distinctive chemical properties because of different number of protons that make their unique identity. One example of isobars are potassium-39 as well as argon-39. each isotope has 39 nucleons inside their nuclei.
They differ with respect to their number of atomic nuclei (potassium-19 and argon-18 respectively). Isobars are crucial in nuclear physics as well as mass spectrometry, as well as astrophysics. They offer insight into the nuclear reaction, elemental abundance as well as cosmic process. As a summary, while isotopes represent variations within elements, isobars investigate commonalities between elements that have similar masses. Knowing these concepts enhances our understanding of the the structure of atoms, their chemical properties and the practical application of these concepts within various industrial and scientific areas.
Importance of understanding Isotopes and Isobars concepts in various fields of science
The understanding of the principles behind isotopes as well as isobars is crucial importance to many fields of research due to their numerous applications and consequences.
These are the most important domains where these ideas are important:
Geology and Archaeology: Isotopes have a significant role to play in radiocarbon dating. It allows scientists to assess the age of artifacts from ancient times as well as fossils and archaeological sites. Geology isotopic analysis helps to understand the history of Earth, its past geochemical processes, and climate as well as aiding in the discovery of natural resources, and also making predictions about changes to the environmental.
Nuclear Physics and Astrophysics: Isotopes are essential to the field of nuclear physics because they regulate the behaviour of nuclei in the atomic scale and aid in understanding the nuclear reaction and particle interactions. Astrophysics isotopes offer insight into the evolution of stars as well as nucleosynthesis and the origins of various elements within the universe.
Isotopic analysis in environmental science aids in tracing the origins and the movement of certain substances within the natural environment, including contaminants, nutrients, as well as water. It can be useful in environmental monitoring, pollutant assessment, as well as knowing the ecology’s dynamics.
Medicine: These include radioisotope-based image analysis (e.g., PET scans) to diagnose illnesses, radiotherapy as a cancer treatment and research studies using tracer to study metabolism and drug interactions in the body.
Agriculture and Food Industry: The use of stable isotopes helps determine the amount of nutrient intake both in animals and plants, which helps ensure optimal practices in agriculture. Furthermore, analysis of isotopes can verify the authenticity and source of food items, preventing food fraud as well as ensuring security of food items.
Climate Science: Isotope analyses of tree rings and marine sediments provide information on the past climate, and helps scientists better understand long-term climate trends as well as fluctuations and changes that drive them. Forensics Isotopic analysis assists in forensic investigations in determining the geographical location of the various substances, including mineral deposits, soil and even human remains helping in criminal investigations, and finding the identities of unknown victims.
Material and Industrial Science Isotopes serve as tracer materials to investigate characteristics of material as well as diffusion rates and chemical reactions that occur in industries, such as metallurgy semiconductors, electronics and more.
The Energy Sector as well as Nuclear Technology: Isotopes are crucial in the generation of nuclear power as well as in nuclear medicine and numerous research and development applications within the energy industry, providing more sustainable energy options and furthering the understanding of science.
Understanding isotopes as well as isobars offers a robust analytical toolbox that spans a broad variety of science disciplines that provide valuable information about the world of nature and driving technological advances in several fields. They allow scientists to discover hidden data and solve difficult problems and take informed choices to improve the lives of people as well as the natural environment.
Definition of isotopes
Toxicity variants of an element share an equal number of protons (atomic number), yet differ in terms of neutron count in their nuclei of an atom’s nucleus.herefore that isotopes of elements have similar chemical properties and are in similar positions in the periodic table. They have different mass due to the differing quantity of neutrons that are present in the nucleus. That means isotopes share the same amount of electrons. They also, therefore they exhibit the same chemical behaviour. Carbon-12 and carbon-14 are similar forms of carbon isotopes; both share six protons but differ in terms of how many neutrons exist between them.
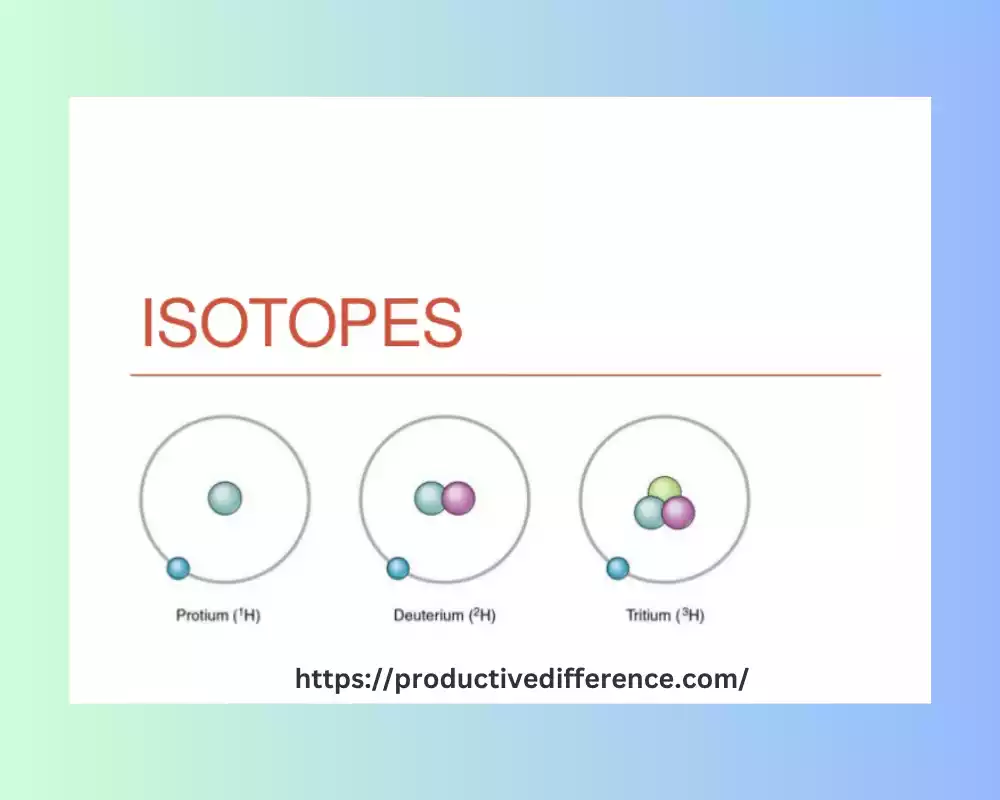
They can be stable or radioactive Isotopes that are stable are in the non-radioactive category and last for long periods, whereas radioactive ones undergo decay, emitting radiation when they change into more stable versions. Analyzing isotopes is crucial for a variety of scientific disciplines, which include archaeology and geology medical chemistry and archaeology and provides valuable information about the physical properties of substances as well as tracing chemical processes and diagnosing medical issues, in addition to many other uses.
Definition of isobars
Isobars are isobars that represent atoms from diverse chemical elements which have identical mass numbers which means they share identical numbers of neutrons and protons in their nuclei of atomic atoms. Although they have differing atomic numbers possess similar masses in atomic form which, in turn, result in similar energies. Similarity in the mass numbers results from the ratio between the numbers of neutrons and protons, that contribute to the overall nucleon number.

Isobars don’t share the same chemical properties to isotopes due to their atomic numbers that are determined by the quantity of protons and protons, differ. Therefore, isobars are located in different places in the periodic table, and exhibit distinct electron configurations as well as chemical properties. Examples include potassium-39 (19 protons 20, 20 neutrons) and Argon-39 (18 protons 21 neutrons) are both isobars possessing 39 nucleons however differing in the atomic number.
Isobars are important in nuclear physics mass spectrometry and Astrophysics because it offers insight into the nuclear reaction, elemental abundance and cosmic events. Isobars assist researchers to understand the commonalities and distinctions among elements of equal masses, resulting in an understanding of the nucleus structure, stability as well as the behaviour of matter within the universe.
Key Differences Between Isotopes and Isobars
The most significant difference between isotopes versus isobars can be seen as follows:
Definition:
Isotopes: They are the different versions of the chemical element contain the same amount of protons (atomic number) however different amounts of neutrons within their nuclei atomic.
Isobars: These are organic atoms from different elements, with the same mass which means they share the exact number of neutrons and protons within their nuclei of atomic atoms.
Composition:
Isotopes:These are the isotopes with identical amounts of protons but differ in the number of neutrons.This creates various mass of atoms.
Isobars: Isobars have the identical quantity of neutrons and protons which results in an identical atomic mass.
Chemical Behavior:
Isotopes: Isotopes from identical elements have the same chemical properties because of similar amounts of protons and electrons which results in the identical electron structure.
Isobars: are diverse elements, each with distinct atomic numbers. Therefore, they possess distinct chemical properties in addition to electron structure.
Position on the Periodic Table:
Isotopes: The isotopes that make up an element are placed together and share the same place within the periodic table.
Isobars: They are diverse elements and appear in various positions within the periodic table.
Symbolic Representation:
Isotopes: They are identified by the symbol of the element after which is the sum of protons as well as neutrons that are in the nucleus. As an example, carbon-12 and carbon-14 are the isotopes for carbon.
Isobars: Isobars can be represented by writing the mass numbers which is followed by the element’s symbol. In this case, potassium-39 and Argon-39 are two isobars possessing a mass value of 39.
Importance and Applications:
Isotopes: Isotopes are a significant application in a variety of disciplines, like radiocarbon dating as well as nuclear medicine, geological studies and environmental dating.
Isobars: Isobars are crucial in nuclear physics and mass spectrometry and Astrophysics. They offer insight into fundamental reactions in the nuclear chain, their abundance and cosmic processes.
Isotopes are referred to the variations within an element while isobars are similarities between elements having similar mass. Understanding the differences between these elements is crucial in a variety of scientific fields and their applications, ranging such as dating old artifacts or studying the deepest levels of the astrophysical and nuclear phenomenon.
comparison chart
Here’s a simplified comparison chart highlighting the key differences between isotopes and isobars:
| Aspect | Isotopes | Isobars |
|---|---|---|
| Definition | Variants of the same element | Atoms of different elements |
| Atomic Nuclei | Same number of protons, different neutrons | Same total protons and neutrons |
| Atomic Mass | Different atomic masses | Same atomic mass |
| Chemical Behavior | Similar chemical properties | Different chemical properties |
| Position on Periodic Table | Same position on the periodic table | Different positions on the periodic table |
| Symbolic Representation | Element symbol + sum of protons and neutrons | Mass number + element symbol |
| Examples | Carbon-12, Carbon-14, Hydrogen-1 | Potassium-39, Argon-39, Calcium-39 |
| Importance & Applications | Radiocarbon dating, Nuclear medicine, Environmental studies | Nuclear physics, Mass spectrometry, Astrophysics |
Significance in Science and Everyday Life
The role of isotopes and isobars in the field of science as well as everyday living is significant, considering that they can be utilized in numerous ways and are essential to studying various natural phenomena.
This is how important they are:
Science and Research:
Isotopes: These are the most important instruments in research. They’re utilized for radiocarbon dating in order to establish the ages of old artifacts and fossils. These provide important insight into the human past as well as the past of Earth. It also aids in understanding the motion of elements throughout ecosystems, giving crucial information on the effects of climate change, pollution as well as natural processes.
Isobars: isobars are vital in the field of nuclear physics research as well as the field of astrophysics. They permit scientists to research the nuclear reaction, stability of nuclear and the abundance of elements found in stars and cosmic processes.
Medicine:
Isotopes: Isotopes are essential uses in the field of the field of nuclear medicine. They allow medical professionals to identify and manage conditions like cardiovascular conditions, as well as neurological diseases with high accuracy. The use of radioisotopes is also used to treat cancer.
Environmental Studies:
Isotopes: Analysis of isotopes helps to track the movements of contaminants and elements within ecological ecosystems. It assists environmental scientists to understand the nutrient cycle as well as water sources and the effect of human actions on the natural environment. This data is essential for the conservation of our environment and sustainable resource management.
Energy Production:
Isotopes: A few isotopes can be employed in nuclear power stations to produce electricity using nuclear fission processes that are controlled.The nuclear industry produces an important portion of electricity used in the world as well as helps in reducing the amount in carbon dioxide that is released.
Agriculture and Food Industry:
Isotopes: Stable can be utilized to measure the amount of nutrients consumed from both plant and animal species that allows scientists to develop better farming methods and increase manufacturing of foods.The analysis of isotopes can also be used to confirm the authenticity of and origins of food items, assuring that food quality and safety are assured.
Forensics:
Isotopes: Isotopic analysis can be used in forensic investigation to identify the geographical origin of certain materials like mineral deposits, soils, as well as human remains. This data aids police investigations, including identifying missing victims and connecting evidence with crime scene.
Industry and Material Science:
Isotopes: Isotopic analyses are utilized to analyze the properties of materials and determine the origin of the raw material, as well as ensure the integrity and authenticity of the products produced from various sectors, like electronics, metallurgy, and gemology.
The conclusion is that isotopes and isobars can have a wide-ranging impact across a variety of disciplines of science and technology. Their importance extends to the field of environmental studies, medicine as well as archaeology and energy production as well as everyday use and make them vital instruments in scientific research in innovation, development, and improvement of the quality of life for humans.
Radioactive Isotopes and Their Applications
Radioisotopes or radioactive isotopes are elemental isotopes which have unstable nuclei which causes the release of radiation when they go through radioactive decay. Their unique characteristics are a great tool with numerous uses in a range of disciplines.
Within the medical field radioisotopes play an important function in the field of diagnostic imaging and treatments for cancer. Radiopharmaceuticals with radioisotopes, including fluorine-18, technetium 99m, and can be administered to patients as part of imaging techniques like SPECT or PET scans. These scans allow for the visualization of the internal organs and tissues and metabolic processes. This aids in early detection and precise diagnosis of illnesses. Radiation with high energy that occurs by decaying isotopes such as cobalt-60 or cesium-137 specifically targets cancerous cells and destroys their cells, while not causing harm to adjacent healthy tissue.
In determining the decay rate of radioisotopes such as carbon-14 or uranium lead isotopes scientists can determine the age of objects as well as reconstruct the timeline of history.
Radioisotopes have applications that go beyond geoscience and medicine. In the industrial setting they can be used for the control of quality and monitoring processes. For example, during manufacturing processes for materials such as plastic, paper and even metal, radioisotopes can be employed to determine the thickness of the product and guarantee consistent quality.
Studies on environmental science benefit by radioisotopes, too. The radioactive tracer helps researchers understand the flow of water within Aquifers, monitor the dispersion of environmental pollutants and track the changes in ecosystem dynamics. These data are crucial to knowing the environmental process in managing resources, as well as dealing with environmental problems.
For scientific research radioisotopes serve as tracer materials to investigate the kinetics of reactions, examine the biochemical pathways, as well as to study the chemical and physical processes. The ability of radioisotopes to release radiation as well as their ability to detect them make them useful in lab settings to conduct various research projects.
Radioisotopes can provide many benefits however, they require careful handling and compliance with guidelines for safety due to the risk of Ionizing radiation. The proper disposal procedures and regulations assure that radioisotopes are utilized in a safe and responsible manner to maximize their capabilities to benefit scientific, medical as well as industrial uses.
Summary
The concepts of isotopes as well as isobars play a major role in understanding the structure of atoms, their chemical properties as well as natural phenomena. Isotopes are variants of the same chemical element that are distinguished by the same amount of protons, but a different number of neutrons. This results in distinct weights of atoms. They offer valuable information to the past as well as present of our planet.
However isobars are elements of various elements which share the same mass numbers that is, they possess the exact amount of neutrons and protons inside their nuclei. Isobars have a major role in nuclear physics, mass-spectrometry as well as astrophysics. They shed the light on nuclear reactions, elements of abundance, as well as the cosmic processes that are shaping the universe.